India's Breakthrough in Cancer Treatment: NexCAR19, The First Homegrown CAR T-Cell Therapy, Gains Approval
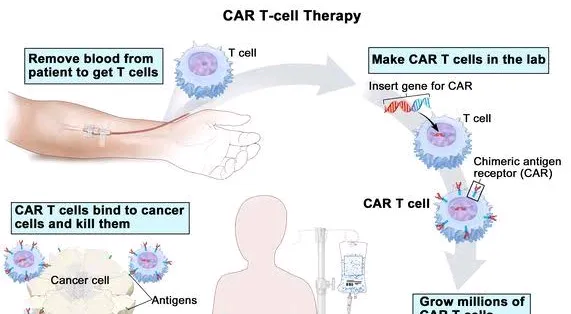
India has achieved a significant milestone in the field of medical science with the development of its first homegrown CAR T-cell therapy, NexCAR19 (actalycabtagene autoleucel), designed to treat relapsed or refractory B-cell lymphomas and leukemia. This innovative therapy was developed through a collaborative effort between scientists at the Indian Institute of Technology (IIT) Bombay, Tata Memorial Hospital, and the industry partner ImmunoACT. The therapy represents a significant advancement in cancer treatment, showcasing India's growing capabilities in biomedicine and the success of the "Make in India" initiative.
In October 2023, India's Central Drugs Standard Control Organization (CDSCO) approved NexCAR19, marking it as the first CAR T-cell therapy available for use in the country. The approval was granted based on the encouraging results from two small clinical trials conducted within India. These trials involved 64 patients suffering from advanced stages of lymphoma or leukemia,...
In October 2023, India's Central Drugs Standard Control Organization (CDSCO) approved NexCAR19, marking it as the first CAR T-cell therapy available for use in the country. The approval was granted based on the encouraging results from two small clinical trials conducted within India. These trials involved 64 patients suffering from advanced stages of lymphoma or leukemia,...